New white paper about Clean in Place (CIP)
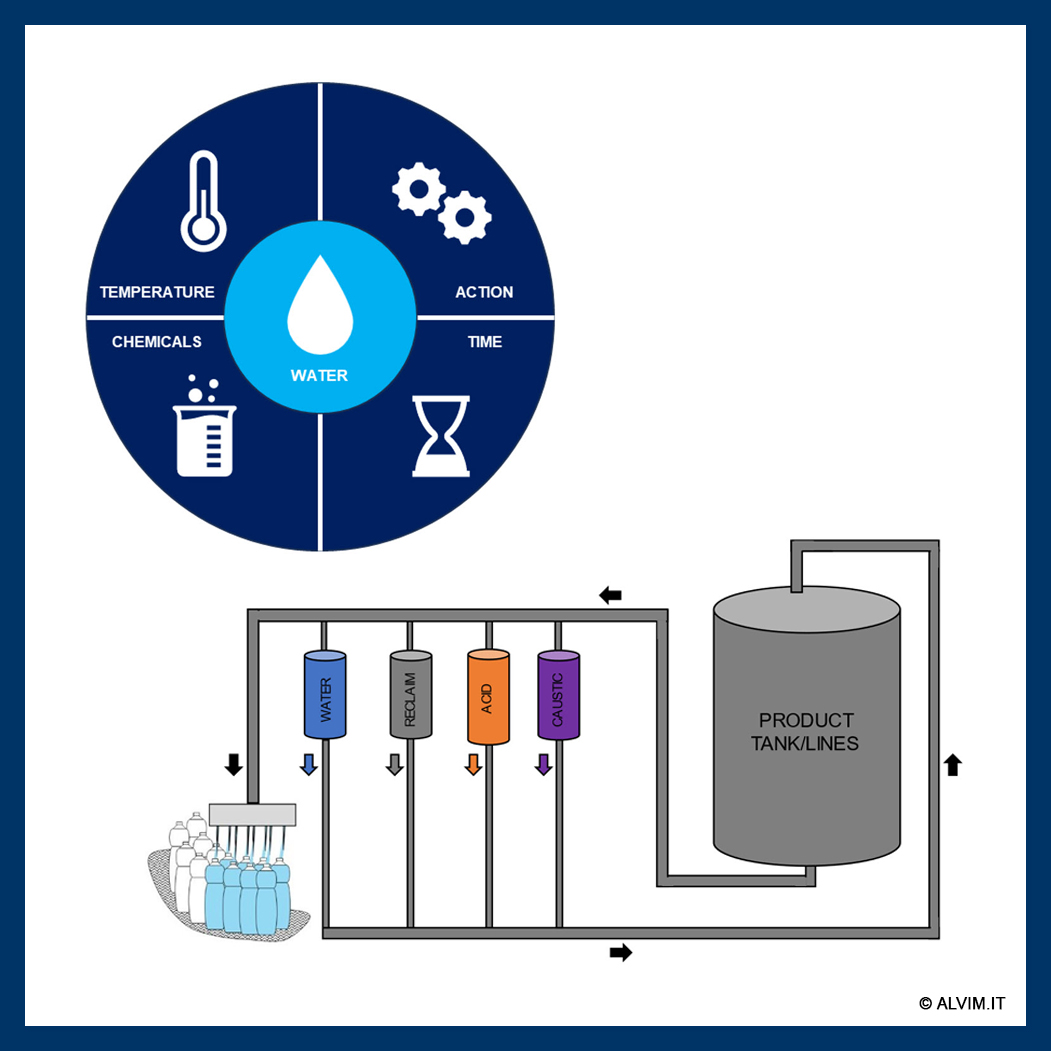
The quality of the final product in many industries is directly linked to the effectiveness of the cleaning process and, consequently, the presence or absence of microorganisms. One of the most practical and widely used methods for cleaning closed processing systems is Clean in Place (CIP)..
Read the full white paper about Clean in Place.